Which of the Following Is a Characteristic of Sodium Ion
Here Niu and co-workers show a design with sodium vanadate hydrate. Potassium depletion will occur whenever.
Rechargeable zinc-ion batteries are promising energy storage devices but suffer from the limited choice of positive electrodes.

. Has a sour taste b. One atom in the bond has a partial positive. The rationale for use of the cyanide.
Ionic compounds form when atoms connect to one another by ionic bonds. Lithium can cause hyponatremia by decreasing sodium reabsorption by the renal tubules leading to sodium depletion. Recently sodiated layer transition metal oxides phosphates and organic compounds have been introduced as cathode materials for SIBs.
Li-ion cells use an intercalated lithium compound as the material at the positive electrode and typically graphite at the negative electrode. Rouelle in the mid-18th century. The electrolyte salt for lithium ion batteries is characteristic of bulky anion that leading to a long separation distance between the ion pair.
Therefore it is essential for patients receiving Lithium treatment to maintain a normal diet including salt and an adequate fluid intake 2500 to 3000 mL at least during the initial stabilization period. Calcium bentonite can be converted to sodium bentonite termed sodium beneficiation or sodium activation by ion exchange process and show many of sodium bentonites properties. When studied in a mammalian expression system the α-subunit of hNa.
An ionic bond is the strongest type of chemical bond which leads to characteristic properties. Cyanide poisoning is detected by the characteristic odor of HCN gas. A lithium-ion battery or Li-ion battery is a type of rechargeable battery composed of cells in which lithium ions move from the negative electrode through an electrolyte to the positive electrode during discharge and back when charging.
Kitamura K Tomita K 2010. Produces H ions in water d. Regulation of renal sodium handling through the interaction between serine proteases and serine protease inhibitors.
The greater the difference the stronger the attraction between the positive ion cation and negative ion anion. In simple form add 510 of a soluble sodium salt such as sodium carbonate to wet bentonite followed by mixing and allow the mixture to leave for a certain time. Sodium channels are the arch-type of voltage-gated ion channels.
10 The human cardiac sodium channel hNa V 15 is a member of the family of voltage-gated sodium channels hNa V 1 to 9. Reaction with air water and hydrogen. Is an electrolyte 2.
Ionic Compound Properties. Decreased tolerance to Lithium has also. As a bleaching agent for domestic use it usually contains 5 sodium hypochlorite with a pH of around 11 it is irritating.
Kellenberger S Schild L. Therefore SIBs are promising next-generation alternatives. Because the electrostatic force is inversely proportional to the square of the separation distance the ionic bond of electrolyte salt with bulky anion is prone to be broken.
Generally elemental sodium is more reactive than lithium and it reacts with water to form a strong base sodium hydroxide NaOHIts chemistry is well explored. Or whether the cyanide is provided with an alternative ferric ion source thereby competitively removing it from the cytochrome using a cyanide antidote kit containing amyl nitrite perles sodium nitrite 10 mL 30 mgmL and sodium thiosulfate 50 mL 250 mgmL. Sodium hypochlorite has a relative density of is 11 55 watery solution.
The usual dietary intake of potassium is 50 to 100 mEq per day. In 1884 Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form. Sodium-ion batteries SIBs are considered as the best candidate power sources because sodium is widely available and exhibits similar chemistry to that of LIBs.
Is named barium hydroxide e. To completely ionize the electrolyte salt the molecules with high. Sodium hypochlorite is a clear slightly yellowish solution with a characteristic odor.
Kleyman TR Carattino MD Hughey RP. Epithelial sodium channeldegenerin family of ion channels. The channel consists of a primary α- and multiple secondary β-subunits.
Sodium is ordinarily quite reactive with air and the reactivity is a function of the relative humidity or water-vapour content of the air. Barbie pegasusun sihri tek parça türkçe dublaj izle Not defteri lisans Eylül barbie pegasusun sihri izle. More recently sodium one of the most abundant elements on earth exhibiting similar physicochemical properties as lithium has been gaining increasing attention for the development of sodium-ion batteries SIBs in order to address the concern about Li availability and costespecially with regard to stationary applications for which size and volume of the battery.
A variety of functions for a shared structure. In chemistry there are three definitions in common use of the word base known as Arrhenius bases Brønsted bases and Lewis basesAll definitions agree that bases are substances which react with acids as originally proposed by G-F. Indicate whether each of the following statements is characteristic of an Arrhenius acid Arrhenius base or both.
Measurements of sodium and potassium ion concentration to introduce the simple concept of quantitation using a calibration curve to highlight the difference between accuracy and precision and illustrate the importance of these factors in clinical assays to practice the correct use of automatic pipettes required for later practical classes 22 Theory and background Note. Potassium is a normal dietary constituent and under steady-state conditions the amount of potassium absorbed from the gastrointestinal tract is equal to the amount excreted in the urine. If the polyatomic ion ends in -ite change to -ous H 2 SO 4 sulfate sulfuric acid H 2 SO 3 sulfite sulfous acid Practice Problems 1.
An active ion transport system maintains this gradient across the plasma membrane.

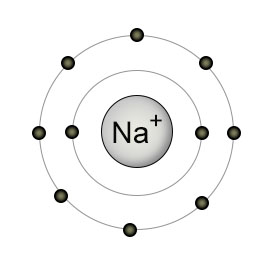
Show Diagrammatically The Electron Distributions In A Sodium Atom And A Sodium Ion And Also Give Their Atomic Number Chemistry Q A

Comments
Post a Comment